Voltar
WEIGHT GAIN AND REPRODUCTION OF Pomacea canaliculata
(Lamarck, 1822) (Gastropoda, Ampullariidae).
*Nara R. Terra1 & Alois Schäfer2
1Fundação Estadual de Proteção
Ambiental Henrique Roessler, RS - Divisão de Biologia
- Porto Alegre - RS - Brasil.
2Fachrichtung Biogeographie - Zentrum für Umweltforschung
- Universität des Saarlandes - Saarbrücken -
Deutshland
*Mailing address: FEPAM - Divisão de Biologia
- Rua Dr. Salvador França, 1707. CEP: 90690 000
Porto Alegre, RS, Brasil. E.mail: labbiofepam@pro.via-rs.com.br
--------------------------------------------------------------------------------
ABSTRACT
Weight gain and reproduction in 40 molluscs,
beginning at 86 days of age, were observed during 373
days. The molluscs were kept in aquaria, with reconstituted
water. They were weighed once a week and at the beginning
of reproductive activity, and egg laying and hatching
were followed daily. The Variation Coefficient as compared
with weight gain is as much as 113%, and the females are
larger and heavier than the males. There is no correlation
between the size of the individuals and sexual maturity.
The females suffer progressive decalcification as they
lay eggs. The individuals die after completing their reproductive
activity. Observations indicate that this species is appropriate
for toxicological trials, because it is easy to breed
in laboratory and costs little to maintain.
Key words: Pomacea canaliculata, reproduction,
growth, South America
--------------------------------------------------------------------------------
INTRODUCTION
Pomacea canaliculata (Lamarck, 1822)
is a species that is widely distributed throughout the
Neotropical region, and occurs all the way from the Amazon
basin in Brazil, to the Province of Buenos Aires, in Argentina.
It is also found in Bolivia, Paraguay and Uruguay (6).
In Rio Grande do Sul the species is found in Passo Fundo,
Marau, Camaquã (PINEDA, personal communication),
in the coastal lagoons (8,12,13), Caxias do Sul, Montenegro,
Gravataí, Porto Alegre and Viamão.
P. canaliculata was introduced for food
purposes initially in China, and extended to Japan, the
Philippines, Korea, Malaysia, Indonesia and Thailand (17).
The habit of living and feeding in the
riparian area, makes P. canaliculata an important species
to provide information regarding changes in the quality
of the aquatic environment.
P. canaliculata was found on Eupatorium
sp., Ludwigia sp., Chara sp. (6) Scirpus californicus
(7). The clutches are constituted by groups of red, spherical,
calcareous eggs (9) and are rarely laid in the water (5,10,16).
Aspects of the biology of P. canaliculata
are referred to by LOPES (14), BACHMANN (1) and CASTELLANOS
& FERNANDEZ (5).
Many species of molluscs have been used
in bioassays with heavy metals, insecticides, herbicides
and other xenobiotics, supplying good results in long-term
tests (2,4,11,19,20). P. canaliculata was used also in
acute tests with heavy metals (8).
The purpose of this study is to provide
the biological foundations for the use of P.canaliculata
in screening tests with toxic substances (TERRA &
SCHÄFER, in preparation).
MATERIAL AND METHODS
The development of P.canaliculata was
observed based on eggs collected at the end of September
at Águas Belas Reservoir in the County of Viamão
(30o02’20"S51o 01’17"W ), RS, Brazil.
The molluscs hatched in the lab were called paternal generation,
and their first generation descendants(F1).
Weight gain, survival and reproduction
were observed for 373 days (between the 86th and 459th
day), in 40 molluscs, distributed in four aquaria, containing
ten specimens each.
The difference between males and females
was established only between adult individuals, when the
egg mass becomes visible in females, and the developed
penis in the males.
The molluscs were distributed in the
aquaria and shelves with the help of the random numbers
table, based on a lot with 300 individuals. The aquaria
(15 cm wide x 30 cm long x 20 cm high) were cleaned with
running water and detergent and rinsed, and were later
washed with 50% nitric acid, rinsed again with running
water, and then with deionized water. The aquaria were
kept covered with a glass plate.
Reconstituted water was placed in the
aquaria; it was prepared daily using deionized water filtered
through activated carbon. Water reconstitution was based
on CABRIDENC (3) and adapted to the hardness found at
the site where clutches were collected (40mgCaCO3). Water
was renewed every 24h, from the birth of individuals until
the end of observations. The water was removed from the
aquaria by siphoning. A PVC cylinder, measuring 10cm in
diameter by 20 cm in length, closed at the lower extremity
by a glass slide, with holes 0.5 cm in diameter over its
length, was used to introduce the water.
The molluscs were fed lettuce ad libitum.
The environmental temperature was maintained
at 22oC ± 1.5oC.
The values of pH, temperature, specific
electric conductivity and oxygen saturation in water were
controlled by daily potentiometric measures before changing
the water.
The individuals of the paternal generation
were weighed weekly on a semianalytical balance (precision
of 0.01g), totalizing 2,120 items of data. Before weighing,
the shells were dried with filter paper. The survival
of individuals was observed every 24 h.
The weight of five males and five females
found copulating in the aquaria was measured to find out
whether there is a relationship of size between sexes
for this activity.
The development of 1,000 F1 individuals
was followed up to the 75th day of life, when they were
weighed and discarded. The F1 individuals were grouped
according to day of birth, in containers with 100 ml of
reconstituted water.
After the clutch hatched, the non-viable
eggs were removed and counted. The survival of the young
was followed by stereoscopic microscopy, by observing
heartbeats.
In order to determine the best fitting
function to the growth data of P. canaliculata , the method
described by RICKLEFS (18) was adopted, using the weight
of the organism as a function of time, based on the von
Bertallanfy equation.
The variation coefficient (VC) was used
to determine weight variability in individuals.
The individuals not selected randomly
for observation were kept in the laboratory for a two-year
period, with the same system for feeding and medium changes,
but were not observed periodically for weight change.
RESULTS
The mean weight gain curve shows two
different stages: the first is described by an almost
linear increase, up to approximately the 18th week of
life (February), when the weight gain difference between
males and females begins; the second extends to close
to the 63rd week of life (January), when weight gain follows
a sigmoid. During the 48th week of life (September), an
impulse in weight gain is observed, coinciding with the
higher weight gain of females, due to the beginning of
the maturation phase of the eggs (Fig. 1).
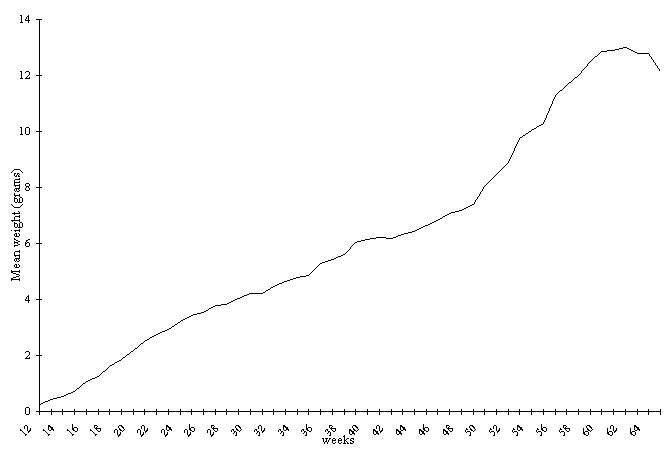
Figure 1. Mean weight gain curves of males and females
P. canaliculata during 52 weeks, beginning in the 12th
week of life at 22±1.50C.
The VC increases until approximately
the 41st week of life (August), and remains stable until
the 48th week which corresponds to the pre-reproductive
period. After this, up to the 62nd week of life (December)
weight loss occurs, coinciding with the reproductive period,
and a new stabilization follows, but with values lower
than the first, identifying the period during which individuals
that have reproduced themselves die. (Fig. 2).
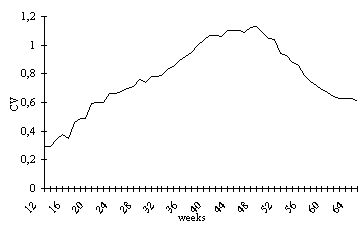
Figure 2. Variation coefficient of weights in P. canaliculata,
during 52 weeks, beginning in the 12th week of life, at
22±1.5°C.
During the observations, the VC ranged
from 29% at the beginning of the experiment to 113% at
the time of maximum weight variation, which corresponded
to the 49th week of life (September). The VC was higher
than 100% for a 13-week period, between the 39th and 52nd
week of life (July to October ).
Utilizing the von Bertallanfy equation
to transform the data ( mean of the weights of 40 individuals),
it was observed that they are described by a straight
line ( r=0.99; n=40) up to 52 weeks of life. Beginning
at that age, the weight gain rose sharply, due to the
maturation of the female gonads and egg formation. During
the development phase, when the individuals are mature,
the conspicuous pink color of the gonads stands out in
the female, giving even the
shell a reddish tint. In males, the penis
is seen to be quite well developed in this phase, like
a whitish formation throughout its length, except in individuals
that have intense sexual activity, when this organ presents
a pinkish tip. During the reproductive period, the females
are larger and heavier than the males. In only a single
pair was it observed that the weight and size of the male
were greater than those of the female. This occurred among
the exemplars that did not mature during the first year
of life but were kept under the same conditions as those
under observation.
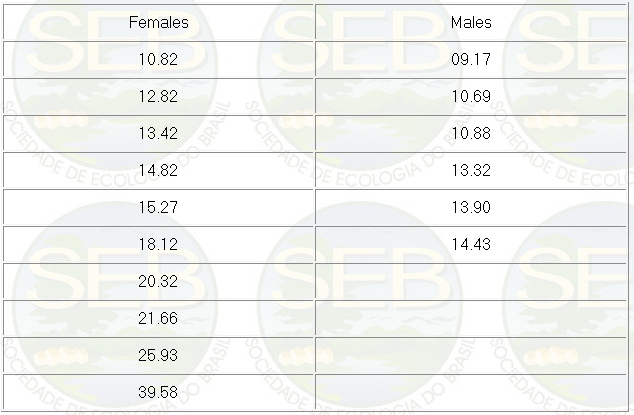
The weight of mature individuals, taking
into account only the males found copulating or with a
pinkish tip of the penis, and females with a visible egg
mass, is presented in Table 1.
Table 1. Absolute weight (grams) of tem
females and six males of P.canaliculata, visibly mature,
observed during weighing. The individuals were not copulating.
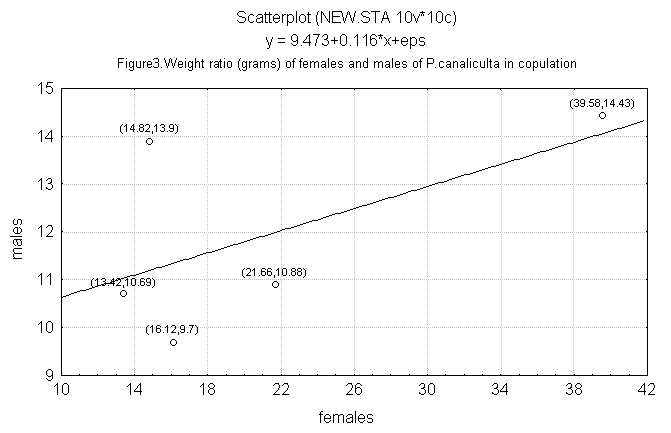
The weight of males and females copulating in the laboratory
does not present a correlation (r = 0.54; n = 5), suggestingthat
there is no relationship of size among copulating individuals
(Figure 3).
This study showed the need to stimulate
the male sexually in order for eggs to be laid, thus sterile
eggs occur due to the lack of egg fertilization, and not
due to lack of copulation. This behavior was observed
when there were only females in one aquarium, and only
males in the other, when no laying activity occurred in
the aquarium containing females. Copulation occurred less
than 30 minutes after five males were transferred to the
aquarium in which there were only females, and five females
to the aquarium in which there were only males. The first
laying occurred less than 24 hours after copulation. The
individuals were redistributed at 11 am one day, and,
at 8 am the next, there were already clutches that hatched
after 23 days.
The laying activity begins when the individuals
are approximately one year old, and they die after the
reproductive period. Among individuals who do not mature
during the first year, only a few lay eggs a few times
during the second year, and die immediately after this.
Most of these eggs were non-viable.
During observations in the laboratory,
the eggs hatched at between 19 and 36 days, and 68% of
them were viable.
It was observed that the retardation
of weight gain in P. canaliculata coincides with the smallest
daily photoperiod.
The clutches laid in water did not hatch.
During the reproductive period, the P.canaliculata
females suffer progressive decalcification as the clutches
are laid, making the shells fragile.
The mean number of eggs laid per female
was six, with an average of 82 eggs per clutch. The survival
determined for F1 individuals up to 75 days of life is
38.6% under controlled laboratory conditions. The mean
weight of individuals at 75 days of life is around 0.06
g, under the same conditions.
DISCUSSION
As to the time of year when reproduction
began and ended, September and February, the results are
different from those mentioned in literature. This difference
may be related to the different latitudes where other
studies were carried out (1, 14). It is observed that
some organisms lay eggs throughout the reproductive period,
while others begin this activity later or end it earlier
(1, 14). Genus reproduction time is variable, with a few
individuals maturing at the beginning of October, laying
eggs until the end of February, while others mature later
(1). The same author observed sporadic laying around September
15 (North of Santa Fé at 32o C) and July 17, (Upper
Paraguay at 10o S).In the same study, that author mentions
that sterile clutches do not change color, but does not
specify whether he performed a test to look at the content
of those eggs. In observations performed in the state
of Rio de Janeiro, it was reported that adult individuals
of genus Pomacea lay eggs from February to March, with
renewed rare laying at the beginning of July. However,
the latter were not viable (14).
There must be sexual stimulus to enable
laying eggs. These mature, within 24 days on average.
Approximately 68% of the eggs were viable. Of the individuals
born in a laboratory, 61.4% died before 75 days of age.
Between the first copulation and the
egg-laying, 24 hours can elapse. This disagrees with the
statement that some specimens copulate once or twice,
laying eggs or not, with others lay their eggs at short
intervals of three days to one week, which is the normal
time between copulation and egg-laying (1) The observations
performed in this study disagree with the bibliography
with states that, in the natural habitat, P. canaliculata
lives from three to four years, but agree that the individuals
die after reproduction (5) This sequence of laying causes
decalcification in the females of P. canaliculata. Calcium
mobility was also observed for Pila virens (Lamarck) in
which the calcium content of the gut gland during hibernation
is 13.2% of the sum total of calcium in the organism,
diminishing to 6.5% during the egg-laying season (15).
Molluscs of the same age, kept in the
laboratory under uniform conditions, present great variation
in size (22), as observed in this experiment.
Laboratory observations identified two
more sensitive phases in the life cycle of individuals:
between September and February ( reproductive period )
and between October and April ( hatching).
The reproductive frequency of the females
is variable, as is the period required for hatching. The
females are larger and heavier than the males. The ontogenic
cycle of P. canaliculata is completed in slightly over
one year.
The information on reproduction , weight
gain, egg viability and survival rate of F1 is essential
to become acquainted with the species for use in toxicological
tests. Due to the easy maintenance in the laboratory and
reproductive viability, P. canaliculata is appropriate
for use in this kind of test.
ACKNOWLEDGEMENTS
To Dr. Rosane Lanzer, for reading critiqueing
and making suggestions for the manuscript, to J.O. Menegheti
MSc., for statistical advice, to Dr. Maria Cristina Pons
da Silva for species identification , to Dr. M. Dolores
S. Pineda, to biologists Rita Semeraro, Eliete Göepfert
and Eunice N. M. Kertész for their help in laboratory
activities.
--------------------------------------------------------------------------------
RESUMO
Ganho de peso e reprodução
em Pomacea canaliculata (Lamarck, 1822) (Gastropoda, Ampullariidae).
Foi observado durante 373 dias, o ganho de peso e a reprodução
em 40 moluscos, a partir de 86 dias de vida. Os moluscos
foram mantidos em aquários com água reconstituída.
foram pesados semanalmente e no início da atividade
de reprodução, acompanhou-se diariamente
a realização das posturas e a eclosão
dos ovos. O coeficiente de variação em relação
ao ganho de peso alcança 113%, sendo as fêmeas
maiores e mais pesadas que os machos. Não há
correlação entre o tamanho dos indivíduos
e a maturidade sexual. As fêmeas sofrem descalcificação
progressiva a medida que efetuam as posturas. Os indivíduos
morrem após cumprida a atividade reprodutiva. As
observações indicaram que esta espécie
é apropriada para realização de ensaios
toxicológicos, devido a facilidade de criação
em laboratório e o baixo custo de manutenção.
--------------------------------------------------------------------------------
REFERENCES
Bachmann, A.C. Apuntes para una hidrobiologia
argentina. II-Ampullaria insularum (Orb) y A. canaliculata
lam. (Moll. Prosobr. Ampullariidae); observaciones biológicas
y ecologicas. Actas y Trabajos do Congresso Sudam. Zool.n01
La Plata, p. 19-24, 1960.
Bluzat; R. & Seugé, J. Effets à long
terme de quatre détersifs chez le pulmoné
d’ eau douce Lymnaea stagnalis L.: intoxication
des animaux dès l’ éclosion. Environ.
Pollut. (series A) 25: 105-122, 1981.
Cabridenc, R. Les bioessais en ecotoxicologie. In: Séminaire
CETESB/ IRCHA, São Paulo-1v.,1979.
Canton, J.H. & Sloof, WThe usefullness of Lymnaea
stagnalis L. as a biological indicator in toxicological
bio-assays (model substance a -HCH). Water Res, 11: 117-121,
1977.
Castellanos, Z. J. A. e Fernandez, D. MOLUSCA, GASTEROPODA,
Ampullariidae. Fauna de agua dulce de la Republica Argentina.
Vol. XV. Moluscos gasteropodos. Fasc. 1, Ampullariidae,
1976.
Cazzaniga, N. J. Pomacea canaliculata (Lamarck,1801) en
Catamarca (Argentina) y un comentario sobre Ampullaria
catamarcensis Sowerby, 1874 (Gastropoda, Ampullariidae).
Iheringia. Sér. Zool (66) : 43-68, 1987.
Cazzaniga, N. J. & Estebenet, A. Effects of crowding
on breeding Pomacea canaliculata (Gastropoda: Ampullariidae).
Comp.Physiol. Ecol. 13 (3): 89 – 96, 1988.
Chomenko L.. Bioindikation und Raumbewertung mit Mollusken
der Familien Ampullaridae und Hydrobiidae, 1986, 221 pp.
Ph.D.Thesis Universität des Saarlandes, Saarbrücken,
Germany.
Hylton-Scott, m. j. Desarrollo embrionário de Ampullaria
canaliculata. Revta. Museo La Plata, 34 : 373-85, 1934.Hylton-Scott,
M.J. Estudio morfologico y taxonomico: de los Ampullarideos
de la Republica Argentina. Rvta. Museo Argentino de Ciências
Naturales Bernardino. Rivadavia 3 (5):229-380, 1957.
Khangarot, B.S. & Ray, P.K. Sensitivity of freshwater
pulmonate snails, Lymnaea luteola L., to heavy metals.
Bull. Environ. Contam. Toxicol., 41: 208-213, 1988.
Lanzer, R.M. Interpretação da distribuição
e ocorrência de moluscos dulce- aquícolos
nas lagoas costeiras da região sul do Brasil. 1983.
Diss. Mestrado CPG Ecologia, UFRGS, Porto Alegre,RS, Brasil.
Lanzer, R.M. & Schäfer. A. Padrões de
distribuição de moluscos dulceaquícolas
como indicadores de condições tróficas
em lagoas costeiras do sul do Brasil. Rev. Brasil. Biol.,
45 (4): 535-545, 1985.
Lopes, H. de S. Sobre Pomacea canaliculata (Lamarck, 1822)
(Mesogastropoda, Architaenoglossa Mollusca).
Rev. Bras. Biol., 16 (4): 535-42, 1956.
Meenakshi, V. Distribution of calcium in the soft parts
of Pila virens (Lamarck) J.Zool. Soc. Índia, 1(1):
35-40, 1955.
Michelson, E. H. On the generic limits in the family Pilidae
(Prosobranchia: Mollusca). Breviora Mus. Comp. Zool. Harvard
Coll (133): 1-10, 1961.
Mochida, O. Spread of freshwater Pomacea snails (Pilidae,
Mollusca) from Argentina to Asia. Micronesica (Suppl.
3): 51-62, 1991.
Ricklefs, R.E. A graphical method of fitting equations
to growth curves. Ecology, 48(6): 978-83, 1967.
Schulte-Oehlmann, U.; Bettin, C.; Fioroni, P.; Oehlmann,
J. & Stroben, E. Marisa cornuarietis (Gastropoda,
Prosobranchia): a potencial TBT bioindicator for freshwater
environments. Ecotoxicology, 4: 373-383, 1995.
Tate. T.M.; Spurlock, F.A. & Christian, F.A. Effect
of Glyphosate on the Development of Pseudosucccinea columella
Snails. Arch. Environm. Contam. Toxicol., 33: 286-289,
1997.
Yager, C.M. & Harry, H.W. The uptake of radioactive
zinc, cadmium and copper by the freshwater snail Taphius
glabratus. Malacologia, 1:339-53, 1964

