Voltar
Herbivory, tannins and sclerophylly
in Chamaecrista linearifolia (Fabaceae) along an altitudinal
gradient
João Augusto Madeira* , Katia Torres Ribeiro,
G. Wilson Fernandes
Ecologia Evolutiva de Herbívoros Tropicais / DBG/
ICB/Universidade Federal de Minas Gerais, CP 486, Belo
Horizonte, MG 30161-970, Brazil
--------------------------------------------------------------------------------
ABSTRACT
The concentration of tannins in leaves,
fruits, and flowers, leaf sclerophylly, leaf size and
herbivore damage in Chamaecrista linearifolia (Fabaceae)
were measured on three populations along an altitudinal
gradient (600m, 850m, and 1,100m), in a cerrado vegetation
in Minas Gerais, southeastern Brazil. Proportion of leaf
area lost to insects was used as an index of herbivore
damage, and its relationship with the other variable was
analyzed. Greater tannin concentrations were found in
leaves, followed by fruits, and flowers, respectively.
With increasing altitude leaves tended to be smaller,
more sclerophyllous and less predated, but there was no
consistent altitudinal change in tannin concentration
in any organ. Damage due to herbivores was related only
to the altitudinal gradient but had no relationship with
any other measured characteristics. The constancy in tannin
concentration along the altitudinal gradient besides large
variation in leaf damage weakens the idea that tannin
production is strongly related to herbivore pressure.
Key words: altitudinal gradient; cerrado; insect herbivory;
sclerophylly; tannins.
--------------------------------------------------------------------------------
INTRODUCTION
Chemical compounds of toxic or digestibility
reducing potential have an important role in plant defense
against herbivores and pathogens (19, 32, 34, 41). Nevertheless,
the synthesis, maintenance and sequestration of these
compounds may be costly to the plant (7, 10, 31). Most
theoretical models on the interactions between herbivores
and plant defense consider that plants should be under
intense selection to produce an optimal defense level
(5, 9, 27, 31, 32). In this cost/benefit relationship,
the production of large amounts of defense of high molecular
weight, such as tannins, would be advantageous
to defend long-lived organs, as observed
when comparing tannin concentration in leaves of different
plant species (9). The rationale is that these compounds
have a high initial cost of production but, in the long
term, they would be cheaper than low molecular weight
substances, due to low turnover rates and maintenance
costs (but see 14). We propose that the same pattern may
occur between organs of the same plant with different
longevities.
Along mountain slopes, variation in abiotic
conditions may affect resource availability and, as a
consequence, the carbon/nutrient balance of plants. With
increasing elevation there is an increase in radiation
intensity; also the soils are generally more exposed and
shallower and have reduced nutrient availability and lower
moisture-retaining capacity (35). If the reduction in
water and/or nutrient availability is not accompanied
by an inhibition of photosynthesis, plants at higher altitudes
should produce an excess of carbon (25). If the production
of secondary compounds is strongly related to resource
availability (6, 25, 39), the concentration of C-based
compounds, such as tannins, should increase at higher
altitudes. Nevertheless, herbivore pressure may be higher
at lower altitudes, as shown by many surveys of insect
herbivore abundance along altitudinal gradients both in
tropical and temperate zone (12, 21, 23, 42, but see 26,
33). Therefore, tannin production may reflect a balance
between herbivore pressure and resource availability.
Tannins are carbon-based compounds of
high molecular weight and are believed to reduce the digestibility
of plant tissues by precipitating the proteins and enzymes
of ingested tissues in the gut of herbivores (27, 31,
38, 41). Tannins also injure the gut of some insects (3,
19). They are viewed as strong anti-herbivory defenses
of widespread effectiveness (19, 31, 32, 38) and seem
to have a dosage-dependent mode of action (18, 19, but
see 20).
Sclerophylly is a leaf trait also closely
related to the nutrient status of the plant (28, 40) and
plants with high C/N ratios are expected to be highly
sclerophyllous (40). However, the role of each evolutionary
force that may lead a species to bear sclerophyllous leaves
is controversial. Sclerophylly may be an adaptation to
water conservation, nutrient conservation and/or prevention
of damage (40). In all cases, plant populations at higher
elevations are expected to be more sclerophyllous. If
sclerophylly is advantageous against herbivory, a negative
correlation between sclerophylly and damage due to herbivory
would be expected, independent of altitude. As tannin
concentration and sclerophylly are similarly influenced
by C/N ratio, we would also expect a positive correlation
between them.
In this study we estimated tannin concentration
in leaves, fruits and flowers, leaf sclerophylly, and
herbivore pressure in three populations of Chamaecrista
linearifolia var. latifolia Barneby (Fabaceae) along an
altitudinal gradient in southeastern Brazil. The following
questions were addressed: 1) Is there any difference in
tannin concentration between leaves, flowers and fruits?
2) Do tannin concentration and leaf sclerophylly increase
with increasing altitude? 3) Does leaf damage by herbivore
increase with decreasing elevation? 4) Are leaf tannin
concentration and sclerophylly positively correlated?
5) How are they related to leaf damage by herbivores?
MATERIAL AND METHODS
Chamaecrista linearifolia var. latifolia
is a perennial herbaceous legume, typical of cerrado and
high elevation vegetation. It has a simple architecture,
with many thin branches emerging from the ground. The
branches rarely bifurcate and each supports around 10
to 20 leaves. Leaves have two leaflets, are resinous and
have high tannin concentration (37).
Study area
The three populations chosen for this
survey are at 600, 850 and 1,100m above sea level, along
on the western slope of a mountain elevated from the Furnas
Dam, Minas Gerais state, southeastern Brazil (20o 40’W,
46o 19’S). It is an ancient terrain with quartzite
preponderance and nutrient-poor sandy soils (22). The
drier months are May to August (1), and this study was
conducted in July 1995.
The 1,100m population was at the mountaintop,
in open shrubby vegetation called "campo rupestre"
(22). It is subject to direct sunlight during all day.
The soils are exposed and erosion is intense, increasing
the soils’ natural poverty. The population at 850m
was on deeper soils, with taller and denser vegetation
coverage. Some shade occurs due to sparse trees of the
savanna (cerrado). The 600m population was near the dam’s
barrage, where the soils were deeper and covered by herbaceous
vegetation. More and taller trees are present, increasing
shade over this population.
Tannin quantification
15 individuals of C. linearifolia were
chosen at random from each population for tannin analysis.
All leaves from three branches chosen at random and all
fruits (of different ages) and flowers of each individual
plant were collected, placed separately in an ice box
and quickly taken to laboratory for tannin bioassay. The
radial diffusion method (17) was used to quantify the
condensed and hydrolisable tannins. The fresh material
was macerated and an extract from 0.3g of each sample
was obtained in 1mL of aqueous 50% methanol. Extracts
were placed in round orifices made on a type I agarose
gel cover containing bovine serum albumin (BSA) in Petri
dishes. Three replicates from each organ were made for
each individual. The precipitation of BSA by the extracted
tannins produces an opaque halo on the gel, the diameter
of which is proportional to the tannin concentration of
the extract. From each halo we took two perpendicular
diameter measures, and used the mean value. Results were
compared to a standard curve obtained for tannic acid
and BSA, hence providing an estimation of tannin concentration
(mg tannic acid g-1 fresh material) (17).
The astringency (protein-precipitation
property) depends on the concentration and also on the
composition of tannins, because there is great variation
in the reactivity of each compound type (20). Therefore,
estimating tannin concentration of an extract using a
standard curve constructed for a standard polyphenol may
lead to error (8). In this work, we estimated astringency
variations within a single plant species, and assumed
that the qualitative composition of its tannins, and consequently
their reactivity, should not vary consistently between
populations or individual plants. Hence, astringency should
be proportional to tannin concentration and provide a
good relative quantification.
The variation in tannin concentration
according to altitude and plant organ was analyzed with
a two-way ANOVA (43).
Sclerophylly and leaf area
Specific leaf weight (SLW=dry weight/area)
was used to estimate leaf sclerophylly (40). Ten mature
leaves per plant were collected from the same individuals
used for tannin quantification (15 plants per altitude).
Leaves were dried at 60oC for 48 hours and then weighed
on an analytical balance. We used a LICOR 3,000-leaf area
meter to measure
leaf area. SLW and leaf area values showed
normal distribution and homogeneous variances, allowing
the utilization of ANOVA (43). Simple linear regressions
were performed between sclerophylly and tannin concentration
within plants of each population to test for positive
correlation between these traits.
Herbivory
Leaf damage by leaf chewers, used as
a herbivory index, was estimated as the proportion of
lost leaf area. Leaf chewing was probably caused by several
insect taxa. Lepidopteran larvae, Orthopteran nymphs and
adult beetles were commonly found feeding on C. linearifolia
leaves. On each population a transect line was extended
and the first ten plants found within a 1m width on both
sides of the line were used to estimate herbivory. On
each individual we measured the total leaf area and the
proportion of leaf area removed by herbivores on 10 old
leaves from 5 branches chosen at random. Only leaves with
signs of both leaflets were considered, to avoid an overestimation
of herbivory, which would occur if abscised leaflets were
considered as eaten. This caution might have lead to underestimation
( 8).
Total and damaged leaf area were estimated
using a grid of 0.25cm2 (0.5 x 0.5cm) drawn on a glass
dish. The number of squares touched by the leaf lamina,
or by its visual reconstitution, was counted, providing
the number of squares containing intact and consumed leaf
lamina for each leaf. Mean total leaf area and mean proportion
of consumed leaf area by herbivory were calculated for
each individual plant. Values were expressed in number
of touched squares. Differences in damaged leaf area between
populations were tested with a Kruskal-Wallis test, as
variances were not homogeneous (43). A stepwise multiple
regression was employed to verify the effects of sclerophylly,
leaf area, leaf tannin concentration and altitude on herbivory.
RESULTS
Tannin concentration
Leaves had significantly higher tannin concentrations
than fruits, while flowers had the smallest tannin concentrations.
This pattern was consistent in all populations. Nevertheless,
tannin concentration within organs did not vary among
populations (two-way ANOVA; Organs: F2,126 = 28994.96,
p < 0.0001; Altitude: F2,126 = 152.79, p= ns; Interaction:
F4,126 = 1.25, p = ns (Figure 1).
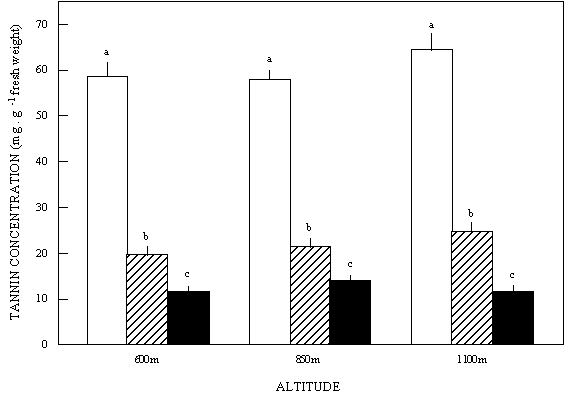
Figure 1: Tannin concentration in leaves fruits and flowers
for the three populations of Chamaecrista linearifolia
(mean ± s.e.). Different letters denote statistically
significant differences. = leaves; = fruits; = flowers.
Sclerophylly and leaf area
Leaves tended to be smaller and more
sclerophyllous at higher altitudes (one-way ANOVA; Leaf
area: F2,40= 14.40; p < 0.0001; Sclerophylly: F2,40
= 19.38; p < 0.0001; Figure 2a,b). There was no significant
relationship between leaf sclerophylly and leaf tannin
content at any population (600m: r2 = 0.041, p = n.s.;
850m: r2 = 0.134, p = n.s.; 1100m: r2 = 0.012, p = n.s.).
The relationship between leaf area and sclerophylly was
weak considering all plants together (y = 0.02 - 0.41x;
r2 = 0.17; p < 0.01; n = 43) and not significant when
each population was considered separately.
Herbivory
The proportion of leaf area consumed by leaf-chewing
herbivores increased with decreasing altitude. Nevertheless,
herbivory was not correlated with leaf sclerophylly, tannin
concentration nor leaf area, and these variables were
excluded from the stepwise multiple regression model (herbivory
x altitude: y = 22.07 - 0.67x; r2 = 0.45; p < 0.0001;
n = 38 (Figure. 2c).
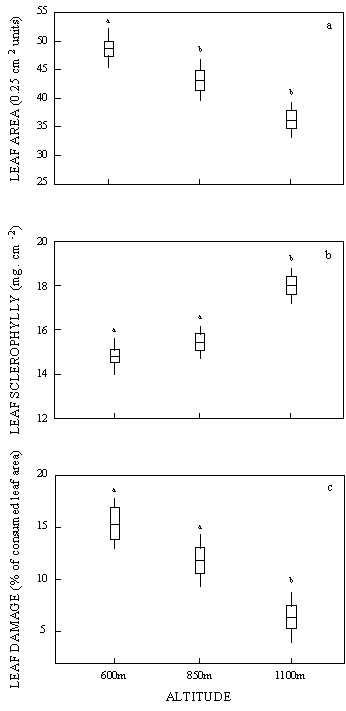
Figure 2: Leaf area, sclerophylly and herbivory (Mean
± s.e. and confidence intervals) on 3 populations
of Chamaecrista linearifolia along an altitudinal gradient.
2a) Leaf area (number of 0.25cm2 squares touched by leaf
lamina); 2b) leaf sclerophylly (specific leaf weight [SLW
= dry weight / area]); and 2c) herbivory (percent of leaf
area [in squares, like 2a] lost due to insect chewing
herbivory).
DISCUSSION
Chamaecrista linearifolia showed increased
levels of specific leaf weight and a reduction in mean
leaf area with increasing altitude. Smaller leaf area
and higher specific leaf weight are two features frequently
associated with sclerophylly (29). Furthermore, plant
scleromorphism tends to be accentuated at higher altitudes,
due to increased radiation intensity and water and nutrients
limitation (15, 35). Nevertheless, there was no strong
correlation between leaf area and sclerophylly considering
plants as units.
In contrast, tannin content within organs
(leaves, fruits, and flowers) did not differ statistically
between the three populations along the altitudinal gradient.
Tannins showed a conservative pattern of higher concentration
in leaves, followed by fruits and flowers, respectively.
Herbivory showed no correlation with
either tannin concentration, sclerophylly or leaf area,
but was clearly more intense at lower altitudes. This
pattern may be related to a decrease in herbivore abundance
with increasing altitude.
The constancy in tannin concentration
across altitudes considering leaves, fruits and also flowers,
and the absence of correlation between tannin content
and herbivore damage suggest that tannin production is
not directly related to herbivore pressure, at least in
this species. The idea of a direct relationship between
herbivory and concentration of defensive compounds such
as tannins is based on the assumption that their production
compromise other plant’s functions (7, 13, 36).
Nevertheless, in plants on poor soils and under high light
intensity, the photosynthetic activity may exceed its
capacity to acquire nutrients, leading to high abundance
of C-based compound (25). This is perhaps the reason for
a luxuriant accumulation of suber by many cerrado tree
species (16, 24). Other studies conducted in sclerophyllous
vegetation describe an unexpected constancy in tannin
concentration, both in plants of a single species occurring
at different habitats at same elevations or at different
altitudes in the cerrado (33) or in Mediterranean plants
submitted to severe browsing and thinning treatments (30).
In such cases, the negative relationship between herbivory
and investment in quantitative defenses may be masked
by the carbon excess (39). Furthermore, carbon excess
is related to many other features that may negatively
affect herbivore performance, as increased leaf toughness
and reduced nutritional quality of tissues (33, 40). The
fact that some insect species are either not affected
or even benefited by tannins (2, 3, 4) may have contributed
to this absence of relationships.
It may be interesting to rescue Feeny’s
(11) ideas on plant apparency to interpret the pattern
of decreasing tannin concentration from leaves to fruits
and then flowers, somehow parallel to a decreasing gradient
in longevity. As leaves are usually more "apparent"
in time, they should deserve higher investments in "quantitative"
defenses, which might deter a wide array of herbivores
(11). Moreover, authors (7) proposed that plants with
longer-lived leaves would be benefited if defended by
C-based compounds of high molecular weight, with an initial
elevated cost of production but low turnover rates. Chamaecrista
linearifolia leaves are more persistent than the fruits,
while flowers are the most ephemeral of the three organs.
This positive relationship between longevity and tannin
content suggests the possibility of an extension of Coley
et al.’s hypothesis to different organs within a
plant. It must be remembered, nevertheless, that a direct
relationship between herbivory and tannin production within
organs was not found.
ACKNOWLEDGMENTS
We thank R. Preszler and I. D. Hodkinson
for the valuable criticism on earlier versions of this
paper, and R. Barneby for kindly identifying our plant
as C. linearifolia. We also thank the logistical support
provided by Furnas Centrais Elétricas S/A, the
assistance of its employees, and the kindness of I. Cruz
(EMBRAPA), who loaned to us the leaf area meter. The study
was conducted during a field course offered by the Pos.
Graduate in "Ecologia, Conservação
e Manejo da Vida Silvestre" (Universidade Federal
de Minas Gerais, MG State) in 1995 and we thank all students
for field and laboratory assistance. The U.S. Fish &
Wildlife Service, CNPq (521772/95-8), and FAPEMIG (1950/95)
supported this study.
--------------------------------------------------------------------------------
RESUMO
Herbivoria, taninos e esclerofilia em
Chamaecrista linearifolia (Fabaceae) ao longo do gradiente
altitudinal. As concentrações de taninos
em folhas, frutos e flores, esclerofilia, tamanho foliar
e pressão de herbivoria em Chamaecrista linearifolia
(Fabaceae) foram medidos em três populações
ao longo de um gradiente altitudinal (600m,
850m, and 1.100m), em uma região
de cerrado em Minas Gerais, Brasil. O dano causado por
herbívoros foi estimado pela proporção
de área foliar perdida, e foi avaliada sua relação
com a esclerofilia, área foliar, concentração
de taninos nas folhas e altitude. Foram encontradas maiores
concentrações de taninos em folhas, seguidas
por frutos e flores. Com o aumento da altitude houve redução
na área foliar e na pressão de herbivoria
e aumento na esclerofilia. Não houve variação
altitudinal na concentração de taninos em
nenhum órgão. Herbivoria não apresentou
correlação com nenhuma das características
medidas nas plantas, apenas com o gradiente altitudinal,
sugerindo que a abundância de herbívoros
em cada local pode estar influenciando os padrões
de herbivoria. A constância altitudinal na concentração
de taninos junto a uma enorme variação em
todas as outras variáveis enfraquece a idéia
de que a produção de taninos está
fortemente vinculada à defesa contra herbívoros.
Palavras chave: cerrado; esclerofilia; gradiente altitudinal;
herbivoria; taninos.
--------------------------------------------------------------------------------
REFERENCES
Antunes, Z.A. Caracterização
climática do estado de Minas Gerais. Informativo
Agro-Pecuário, 12: 9-13, 1986.
Berenbaum, M.R. Effects of tannins on growth and digestion
in two papilionids. Entomol. Exp. et Appl., 35: 245-250,
1983.
Bernays, E. A. Tannins: an alternative viewpoint. Entomol.
Exp. et Appl., 24: 44-53, 1978.
Bernays, E.A.; Woodhead, S. Plant phenols utilized as
nutrients by a phytophagous insect. Science, 219: 201-202,
1982.
Berryman, A. A. Towards an unified theory of plant defenses.
In W.J. Mattson, J. Levieux and C. Bernard-Dagan (eds.).
Mechanisms of Woody Plant Defenses against Insects: Search
for Pattern. Springer-Verlag, New York, 1988, p. 39-55.
Bryant, J. P.; Chapin III, F.S.; Klein, D.R.. Carbon/nutrient
balance of boreal plants in relation to vertebrate herbivory.
Oikos, 40: 357-368, 1983.
Coley, P.D. Costs and benefits of defense by tannins in
a neotropical tree. Oecologia, 70: 238-241, 1986.
Coley, P. D.; Aide, T. M. Comparison of herbivory and
plant defenses in temperate and tropical broad-leaved
forests. In: P.W. Price; T.M. Lewinsohn; G.W. Fernandes;
W.W. Benson (eds.). Plant Animal Interactions: Evolutionary
Ecology in Tropical and Temperate Regions. Wiley, New
York, 1991, p. 25-49.
Coley, P. D.; Bryant, J. P.; Chapin III, F. S. Resource
availability and plant herbivore defense. Science, 230:
895-899, 1985.
Feeny, P.P. Seasonal changes in oak leaf tannins and nutrients
as a cause of spring feeding by winter moth caterpillars.
Ecology, 51: 565-581, 1970.
Feeny, P.P. Plant apparency and chemical defense. Rec.
Adv. Phytochem., 10: 1-40, 1976.
Fernandes, G. W.; Price P. W. Biogeographical gradients
in galling species richness: test of hypotheses. Oecologia,
76: 161-167, 1988.
Folgarait, P. J. and Davidson, D. W. Antiherbivore defenses
of myrmecophytic Cecropia under different light regimes.
Oikos 71: 305-320, 1994.
Gershenzon, J., Murtagh, G.J.; Croteau, R. Absence of
rapid terpene turnover in several diverse species of terpene-accumulating
plants. Oecologia, 96: 583-592, 1993.
Giulietti, A.M.; Menezes, N. L.; Pirani,
J. R.; Meguro, M.; Wanderley, M. G. L. Flora da Serra
do Cipó, Minas Gerais: caracterização
e lista de espécies. Bol. Bot., 9: 1-152, 1987.
Goodland, R. Oligotrofismo e alumínio no cerrado.
In: M.G. Ferri (Ed.). Simpósio sobre o Cerrado
III. Edgard Blucher, São Paulo, 1971, p. 44-60.
Hagerman, A.E. Radial diffusion method for determining
tannin in plant extracts. J. Chem. Ecol., 14: 1789-1805,
1987.
Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein
interactions. J. Biol. Chem., 256: 4494-4497, 1981.
Harborne, J.B. Introduction to Ecological Biochemistry.
Academic Press, London. 4th ed. 1993, xii+318 pp.
Haslam, E. Plant polyphenols (syn. vegetable tannins)
and chemical defense - a reappraisal. J. Chem. Ecol.,
14: 1789-1806, 1988.
Janzen, D. H. Sweep samples of tropical foliage insects:
effects of season, vegetation types, elevation, time of
day, and insularity. Ecology, 54: 687-708, 1973.
Joly, A.B. Conheça a Vegetação Brasileira,
EDUSP/Polígono, São Paulo, 1970, 181p.
Lawton, J. H.; MacGarvin, M; Heads, P.A. Effects of altitude
on the abundance and species richness of insect herbivores
on bracken. J. An. Ecol., 56: 147-160, 1987.
Lopes, A.S.; Cox, F.R. Cerrado vegetation in Brazil: an
edaphic gradient. Agron. J., 69: 828-831, 1977.
Mattson, W. J. Herbivory in relation to plant nitrogen
contents. Ann. Rev. Ecol. Syst., 11: 119-161, 1980.
McCoy, E. D. The distribution of insects along elevational
gradients. Oikos, 58: 313-322, 1990.
McKey, D. 1979. The distribution of secondary compounds
within plants in: Rosenthal, G.A. and Janzen, D.H. (eds.).
Herbivores: Their Interaction with Secondary Plant Metabolites.
Academic Press, Orlando, 1979, p. 55-133.
Medina, E.; Garcia, V.; Cuevas, E. Sclerophylly and oligotrophic
environments: relationships between leaf structure, mineral
nutrient content, and drought resistance in tropical rain
forest of the upper Rio Negro region. Biotropica, 22:
51-64, 1990.
Mooney, H.A.; Dunn, L.E. Convergent evolution of Mediterranean-climate
evergreen sclerophyll shrubs. Evolution, 24: 292-303,
1970.
Perevolotsky, A. Tannins in Mediterranean woodland species:
lack of response to browsing and thinning. Oikos, 71:
333-340, 1994.
Rhoades, D. Evolution of plant chemical defense against
herbivores in: Rosenthal, G.A.; Janzen, D.H. (eds.). Herbivores:
Their Interaction with Secondary Plant Metabolites. Academic
Press, Orlando, 1979, p. 3-54.
Rhoades, D.; Cates, R.G. Toward a general hypothesis of
plant antiherbivore chemistry. Rec. Adv. Phytochem., 10:
168-213, 1976.
Ribeiro, S.P.; Silva, C.H.L.; Fernandes, G.W. Habitat,
herbivory and leaf polyphenol concentration in several
melastomataceae native to savannas of Brazil. Journal
of Tropical Ecology (in press), 1997.
Rosenthal, G.A. and Janzen, D.H. Herbivores: Their Interactions
with Secondary Plant Metabolites. Academic Press, Orlando,
1979, xii + 718 p.
Sarmiento, G. Ecological features of climate in high tropical
mountains. In: Vuilleumier, F.; Monasterio, M. (eds.).
High Altitude Tropical Biogeography. Oxford, New York,
1986, p. 11-45.
Seigler, D.; Price, P.W. Secondary compounds in plants:
primary functions. Am. Nat., 110: 101-105, 1976.
Silva, I. M.; Borges, J.C.; Brina, A.E.; Camargo, S.L.;
Costa, A.R.G.; Marques, A.R.; Passamani, M.; Silva, L.V.C.
Taninos em Chamaecrista spp.: efeitos da altitude e localização
na planta. In: Fernandes, G.W. (ed.)
Ecologia e Manejo da Vida Silvestre (Furnas),
Belo Horizonte. unpublished report, 1994, p. 5-19.
Swain, T. Tannins and lignins. In: Rosenthal, G.A. and
Janzen, D.H. (eds.). Herbivores: Their Interaction with
Secondary Plant Metabolites.
Academic Press, Orlando, 1979, p. 657-682.
Tuomi, J., P. Niemelä, F. S. Chapin
III, J. P. Bryant; S. Sirén. Defensive responses
of trees in relation to their carbon/nutrient balance.
In: Mattson, W.J.; Levieux, J.; Bernard-Dagan, C. (eds.).
Mechanisms of Woody Plant Defenses Against Insects. Springer-Verlag,
New York, 1988, p. 57-72.
Turner, I. M. Sclerophylly: primary protective? Func.
Ecol., 8: 669-675, 1994.
Whittaker, R.H.; Feeny, P.P. Allelochemics: chemical interactions
between species. Science, 171: 757-770, 1971.
Wolda, H. Altitude, habitat and tropical insect diversity.
Biol. J. Linn. Soc., 30: 313-323, 1987.
Zar, J.H. Biostatistical Analysis. 2nd ed., Prentice Hall,
Englewood Cliffs, N.J., 1984, 718 p.

