Voltar
USE OF INORGANIC (NPK) AND THE CHU12 MEDIUM FOR
CULTIVATION OF Ankistrodesmus gracilis IN LABORATORY
Lúcia Helena Sipaúba-Tavares, Luciele Cristina
Pelicioni & Alfredo Olivera
1 Centro de Aqüicultura - Universidade Estadual
Paulista - Jaboticabal - SP - Brasil
ABSTRACT
Freshwater microalgae, Ankistrodesmus
gracilis, were grown using two different media: a CHU12
nutrient medium and an alternative, less expensive medium,
the chemical fertilizer NPK. The NPK fertilizer was used
in different proportions (20:5:20, 4:14:8, 12:6:12, and
10:10:10). Similar growth was observed when using the
CHU12 medium and the NPK fertilizer, but only in the proportion
20:5:20. When using the NPK fertilizer in other proportions
the growth performance was lower. The alkalinity, inorganic
carbon and nutrients were found to be very similar for
the different proportions of NPK fertilizer used, but
when using CHU12 these parameters were lower, except for
conductivity and nitrate. The results showed that NPK
in the 20:5:20 combination can be used directly for successful
mass cultivation of A. gracilis.
Key words: culture, Ankistrodesmus gracilis, NPK, CHU12,
limnological parameters.
--------------------------------------------------------------------------------
INTRODUCTION
Inorganic fertilizers have been used
more and more in aquaculture over the last few years as
a way of increasing microalgae biomass.
With this in mind, this work aimed to
study the use of a chemical fertilizer, NPK, in different
proportions, and a CHU12 standard medium (6), which contains
inorganic nutrients and is widely used to cultivate a
species of chlorophycean algae, the Ankistrodesmus gracilis.
This microalgae is commonly used to feed invertebrates
and fish larvae (16).
The use of a complex medium with a low
bacteria count is necessary to cultivate microalgae on
a large scale. The water used must be treated by microfiltration
and/or ultra-violet irradiation. The main requirement
for any nutrient to be used in a mass production program
is to be easily obtained in adequate quantities and have
a low cost (8).
The need to improve the cultivation
of microalgae used for the nutrition of invertebrates
and fish larvae has generated many practical studies in
different fields. These studies are not only of nutrition
but also of the use of alternative media with low costs.
Alternative media should allow efficient production of
algae in the laboratory, and the algae should provide
nutrition to various aquatic organisms.
These fertilizers have been shown to
be efficient for plankton production, especially microalgae,
which form the base of the food chain (2). Laboratory
cultivation of microalgae is a very efficient way to obtain
a specific diet as algal cells have an exponential growth
rate and a high photosynthetic capacity, where protein
is the main product of their photosynthesis.
In the laboratory, under controlled
conditions, the variations in metabolic activity and in
the production of metabolic products are very small. The
microalgae growth only depends on the intrinsic properties
of the cells, assuming that laboratory conditions are
adequate.
Algae can be used directly for the nutrition
of larvae or indirectly to feed several species of zooplankton
which, subsequently, provide food for the cultivation
of fish, shrimp and frogs. When all of the above factors
are considered, studies that develop the techniques of
mass microalgae production are of extreme importance.
MATERIAL AND METHODS
Microalgae Cultivation:
The microalgae were cultivated, over
14 consecutive days, in a 2 liter Erlenmeyer flask illuminated
from above by a 5200 lux, "daylight", fluorescent
light. The algal inoculum was obtained from the Laboratory
of Algae Physiology at the Botanical Department of the
Federal University of São Carlos (SP, Brazil) and
belongs to the algal bank that corresponds to the 005
CH strain.
Two different media were used for cultivation:
CHU12 (6) which is appropriate for the vigorous growth
of species that come from eutrophic environments, and
the chemical fertilizer NPK (mass ratio 20:5:20, 12:6:12,
10:10:10 and 4:14:8) as powder and granulated. About 0.7g
of fertilizer was added to 2L of the culture. The CHU12
medium was prepared according to CHU (6). The NPK fertilizer
proportions were chosen in aleatory form including the
combination of 20:5:20 that was usually found in the works
for cultivation of freshwater algae in laboratory. Three
replicates were used for each treatment and were kept
under constant aeration.
The culture growth was followed closely
to determine the variation of the number of cells throughout
the period of growth. A Neubauer chamber was used for
counting the cells. A growth curve was produced for each
treatment and adjusted to a logistic model to discriminate
between the different phases of growth. The growth rate
was obtained using the exponential phase of the growth
curve, representing the number of cellular divisions per
day, and duplication time, also denominated division time
or generation time, and was calculated from results obtained
from the growth rate, according to Stein (17).
Morphological and Chemical Characteristics
of the Cultivated Microalgae:
All measurements were obtained during
the exponential phase of the growth curve for the cultures.
Total length: it was estimated using
50 algal cells measured by a micrometer eyepiece with
a magnification of 100X.
Biovolume: it was calculated from mean
cell dimensions using the most common form (elongated
cell) for Ankistrodesmus gracilis, and was measured by
calculating the volume of two cones. The formula is given
in equation 1 (4,18,19).
(1) V = ((p .r2.h) . 2) / 3
where: V = cellular volume; r = base
radius of cone; h = height of cone
Dry weight: the dry weight corresponds
to the weight of the totally dehydrated body. It was determined
by taking 10mL from each culture with a density of 15
x 106 cells/mL. These samples were filtered through a
fiberglass filter (GFC 1.2m m pore size), previously washed
in distilled water, under vacuum. Afterwards, the filter
was dried at 60oC until reaching constant weigh (20).
Organic Carbon: the elemental weight
in terms of total organic carbon was obtained from the
relationship between the carbon content and cellular volume
proposed (14) for fresh water microalgae. The formula
is given in equation 2.
(2) C = 0.1204 . V1.051
where: C = organic carbon content in
pg/cell; V = cellular volume
chlorophyll a : it was determined according
to the technique described in literature (7) and the solvent
used was 90% cold acetone, without phaeophytin correction.
Physical and Chemical Analysis of the
Culture Media:
To evaluate the effect of the fertilizer
in the cultivated medium, some physical and chemical variables
were calculated. The analyses were made on alternate days,
as:
Temperature: determined by a digital
Corning PS 16 thermometer.
pH: determined by a digital PS 17 pH
meter.
Conductivity: determined by a digital
Corning PS 15 conductivity meter.
Alkalinity and inorganic carbon (7,10).
Nutrients: analysed using a spectrophotometer
for ammonium (9) and nitrite, nitrate, orthophosphate
and total phosphorous (7).
Dissolved Oxygen: determined according
to the Winkler technique (7).
Variance analysis was applied to the
results (ANOVA, P<0.05) and the Tukey (P<0.05) and
the Duncan (P<0.05) tests were used to discriminate
between the differences found.
RESULTS AND DISCUSSION
In the last few years the food quality
used for cultivation has been questioned, and many alternatives
for improving nutritional values as well as the quantity
directly available to shrimp and fish larvae have been
developing in a more intensive way. Among the alternatives,
inorganic fertilizers (NPK) have become the main source
of research (16).
Cultures of nominally the same species
often showed wide variations in cell morphology and growth
rates. Relating to growth rate and cell density (Figure
1) the NPK 20:5:20 and 4:14:8 (granulate) treatments showed
the best results (p<0.05), with 0.22 and 0.20 div/day,
respectively (Table 1).
Table 1: Mean data and standard deviation for growth
and morphological characteristics of Ankistrodesmus gracilis
algae in CHU12 and NPK (20:5:20; 4:14:8; 12:6:12; 10:10:10)
media.
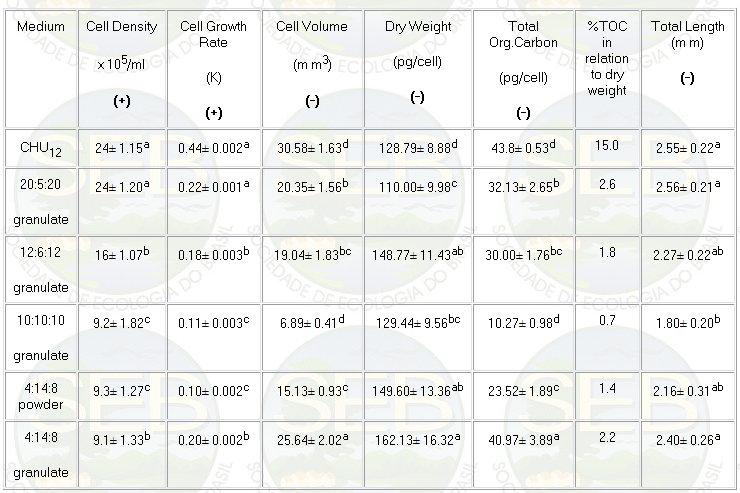
abcd : there are significant differences (p<0.05)
(+) Differences found using ANOVA (p<0.05) and the
Duncan test
(-) Differences found using ANOVA and the Tukey test
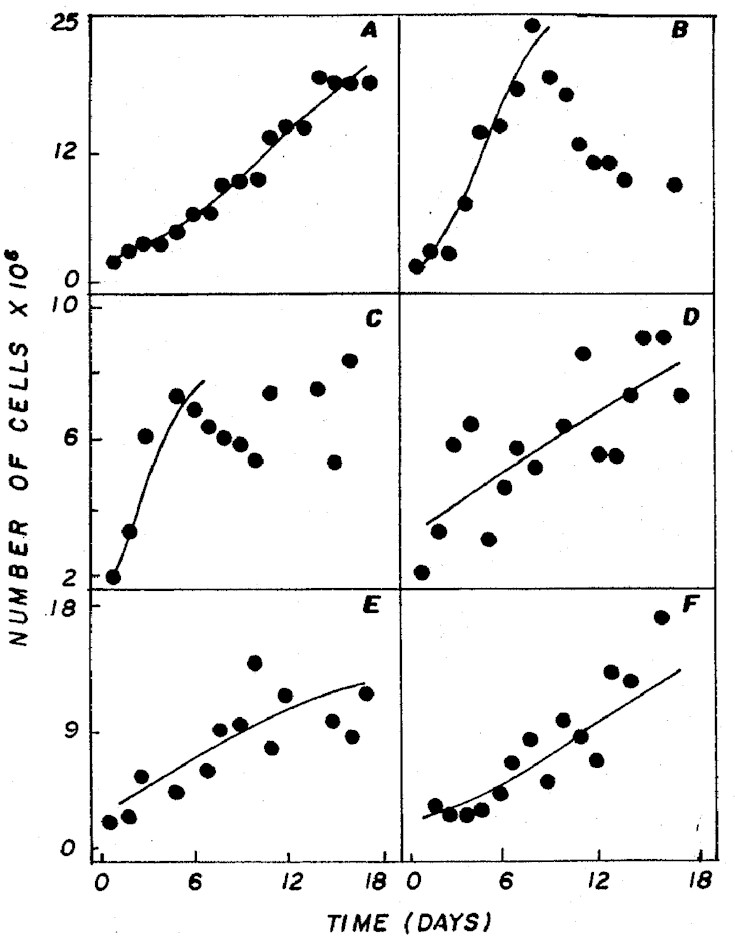 .
.
Figure 1: Cell density of Ankistrodesmus gracilis in
NPK (A= 20:5:20 - granulate; C=10:10:10 - granulate; D=
4:14:8 - powder; E= 4:14:8 - granulate; F= 12:6:12 - granulate)
and CHU12 (B) media.
Comparing the standard CHU12 medium
with the one that showed the best results among the different
combinations of NPK (20:5:20), there were no significant
differences (p<0.05) with regard to cell density. However,
for the growth rate, CHU12 with 0.44 div/day was higher
than NPK (20:5:20) with 0.22 div/day. This can be reflected
by the chlorophyll a levels (Figure 2), found to be similar
in CHU12 and in the combination 20:5:20, while the other
treatments had lower concentrations.
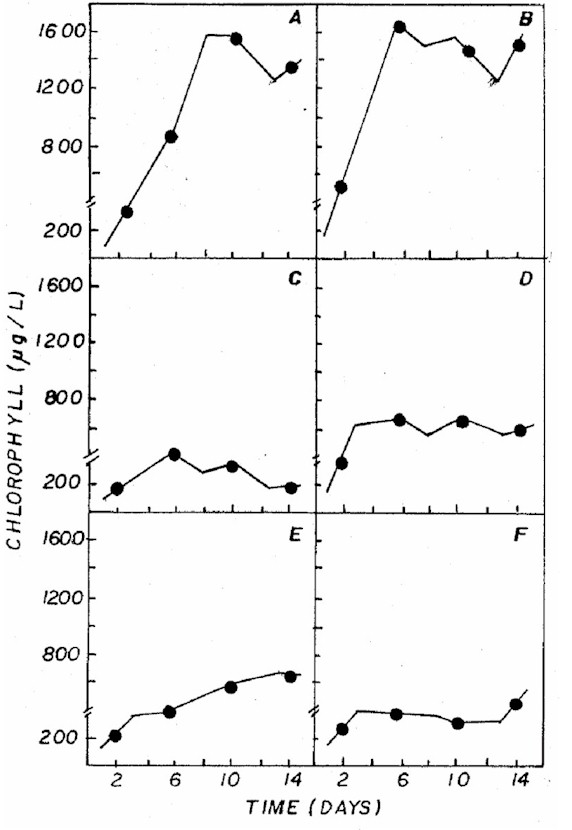
Figure 2: Fluctuation of chlorophyll a (not corrected
for phaepigment) in Ankistrodesmus gracilis microalgae
in NPK (A= 20:5:20 - granulate; C= 10:10:10 - granulate;
D= 4:14:8 - powder; E= 4:14:8 - granulate; F= 12:6:12
- granulate) and CHU12 (B) media.
The total length and cell volume (Table
1) were much smaller than the values found in other research
for the same species of microalgae at the same conditions
(16). The microalgae metabolism variations, when compared
to the components of the medium used in culture, act upon
these characteristics (1).
With regard to total length, significant
differences were not found (p<0.05) between the different
treatments with the exception of the 10:10:10 combination.
With regard to cellular volume, the best treatments were
the CHU12 and the granulated 4:14:8 combination (p<0.05).
For morphological variables the granulated 4:14:8 combination
showed best values (Table 1).
The differences in the chemical composition
of algal cells could be associated to alterations that
occur in the growth phase or the source of available nutrients
(16). The percentage relationship between total organic
carbon and dry weight (Table 1) was greater for the CHU12
(15%) than for the inorganic fertilizer, with a 0.7 to
2.6% variation between the combinations used.
The nature and concentration of the
culture medium influence the carbon content. Scenedesmus
brasiliensis showed different values of carbon per unit
volume for the same stock, when it was cultured in media
of different concentration (14). The carbon level is an
important measure that characterizes the nutritional value
of algae, considered a good indicator of the quality of
algal nutrients.
The maintenance of the culture in the
laboratory with regard to luminosity, temperature and
aeration is fundamental for the development and exponential
growth of algae. Temperature affects the metabolism, the
growth rates and the cellular components of the organisms
(11). Temperature in the cultivation room was 22 + 1oC,
similar to the temperatures noted by others authors (2,
16).
The aeration was constant and the low
presence of bacterial cells was of little importance since
they can be used as a food source by the zooplankton,
which is a direct consumer of phytoplankton.
The aeration of the different culture
media influenced pH, alkalinity and inorganic carbon (Figures
3, 4, 5). Aeration maintains the cells in suspension,
allowing identical growth and assures the inorganic carbon
supply, besides stabilizing the pH. Aeration also increases
the surface culture medium, favoring gas exchanges and
adding CO2 to the medium (13).
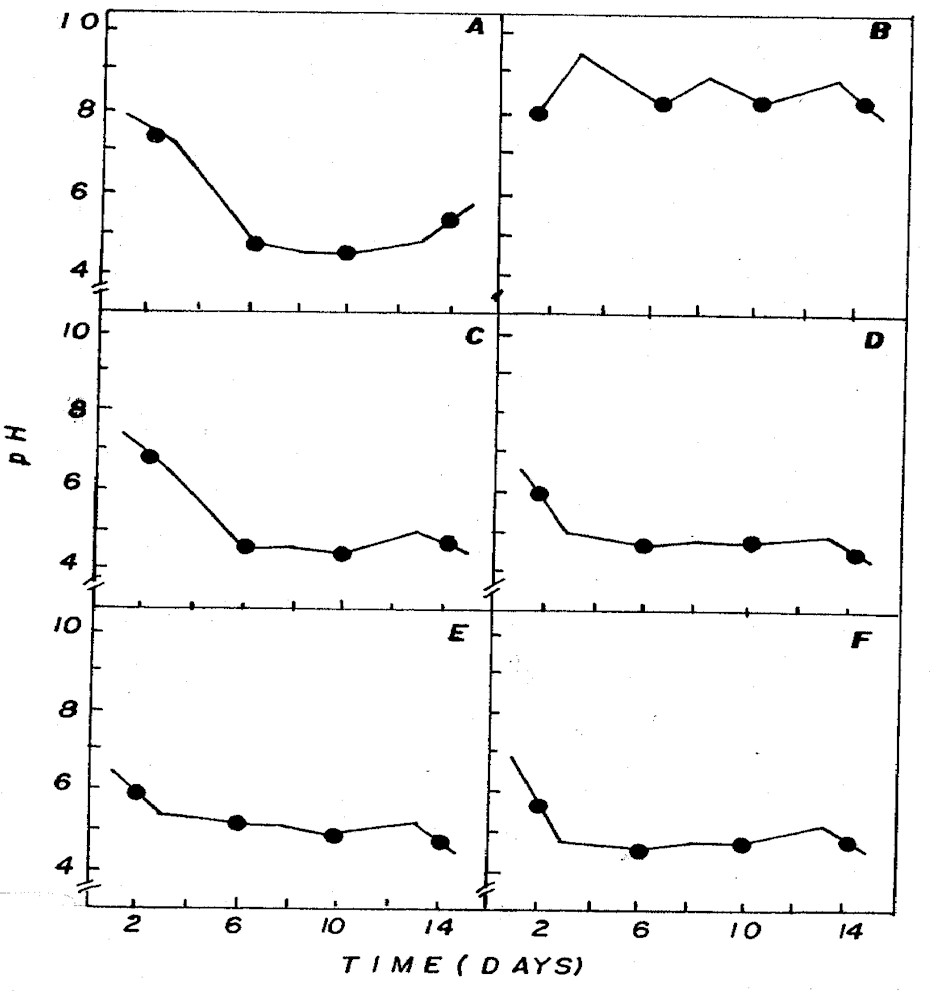
Figure 3: Fluctuation of pH in NPK (A= 20:5:20 - granulate;
C= 10:10:10 - granulate; D= 4:14:8 - powder; E= 4:14:8
- granulate; F= 12:6:12 - granulate) and CHU12 (B) media.
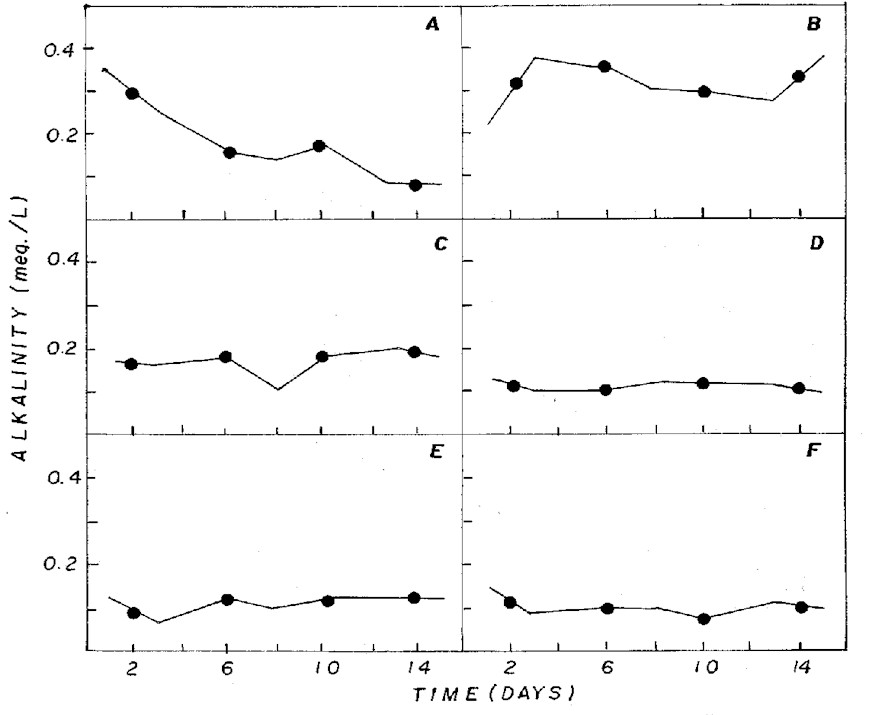
Figure 4: Fluctuation of alkalinity in NPK (A= 20:5:20
- granulate; C= 10:10:10 - granulate; D= 4:14:8 - powder;
E= 4:14:8 - granulate; F= 12:6:12 - granulate) and CHU12
(B) media.
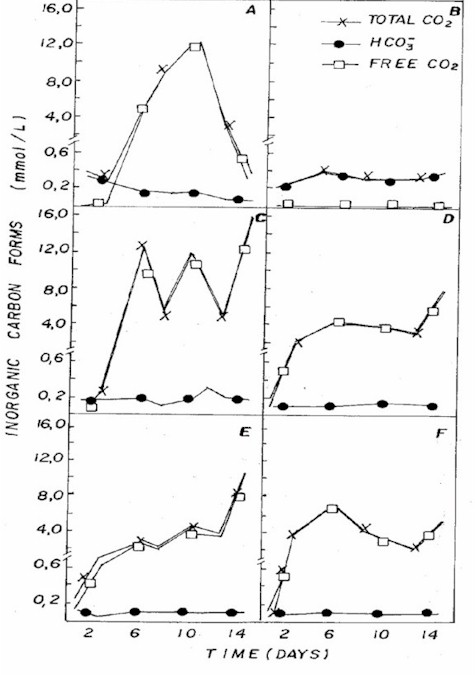
Figure 5: Fluctuation of different forms of inorganic
carbon in NPK (A= 20:5:20 - granulate; C= 10:10:10 - granulate;
D= 4:14:8 - powder; E= 4:14:8 - granulate; F= 12:6:12
- granulate) and CHU12 (B) media.
pH in different combinations was usually
below 7 (Figure 3), being associated to the dominance
of CO2 in the medium (Figure 5) that, according to Moss
(12), is one of the different forms of carbon used by
some algae for photosynthesis in acid pH condition.
In CHU12 medium, bicarbonate was the
predominant form of inorganic carbon (Figure 5), being
associated to pH (Figure 3) that oscillated between 7.8
and 9.4. The inorganic carbon levels were greater in the
NPK combinations. Most algae cells take up carbon dioxide,
which diffuses readily across all membranes, when freely
available. At lower external concentrations, algae may
either be limited or they use bicarbonate as the carbon
source (13).
The CO2 system in natural water plays
a large part in determining the qualitative composition
as well as the photosynthetic activity of the freshwater
phytoplankton (13). The growth rate in the CHU12 was higher
with higher organic matter synthesis, which may lead to
pH increase and consequently CO2 and phosphate decrease
due to these compounds assimilation.
Different combinations of NPK also showed
the highest values for conductivity (Figure 6) when compared
to the standard CHU12 medium, due to higher availability
of nutrients, particularly ammonium (Table 2). Nutrient
concentration is not usually the best factor for estimating
the phytoplanktonic "status" because deficient
cells can obtain nutrients in excess for their immediate
growth (5).
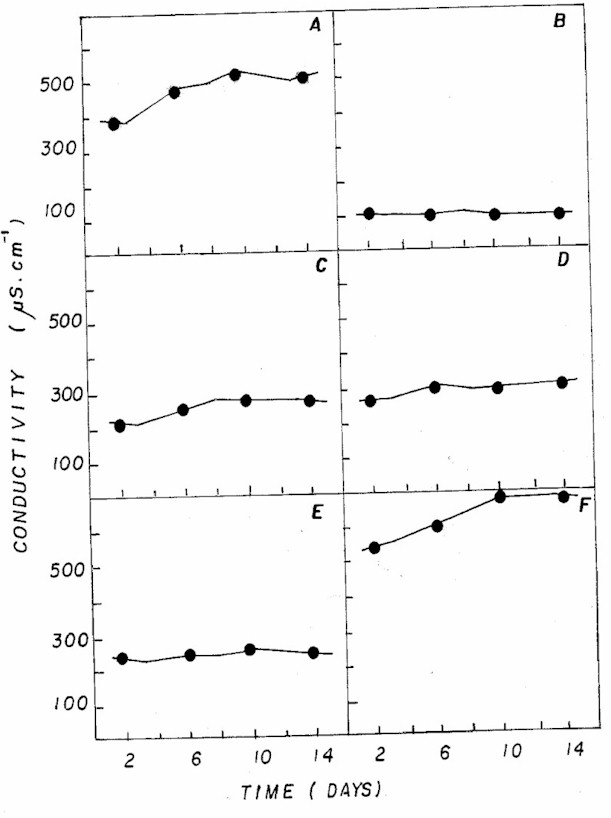
Figure 6: Fluctuation of electrical conductivity in NPK
(A= 20:5:20 - granulate; C= 10:10:10 - granulate; D= 4:14:8
- powder; E= 4:14:8 - granulate; F= 12:6:12 - granulate)
and CHU12 (B) media
With regard to the different nitrogenous
compounds (Table 2), ammonium was dominant, followed in
much lower concentrations by nitrate, which can be assimilated
by microalgae (3).
Table 2: Average values, during the experiment,
of nutrients (µg/L) in the algal culture for different
NPK combinations and with the CHU12 medium.
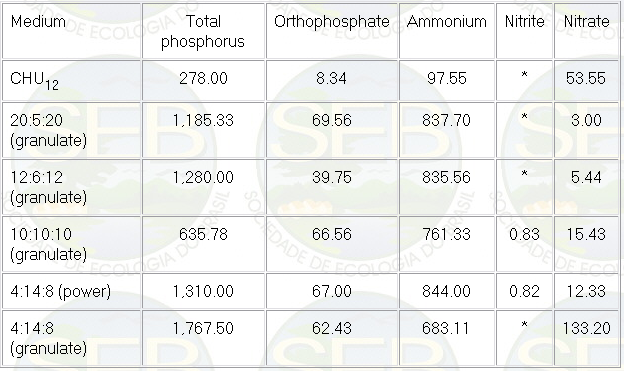
* below detection limit
The low concentrations of nitrate and
nitrite could be also associated to the nitrification
process in general, which is low even in optimal conditions
(5). Nitrite was almost totally absent in some of the
treatments.
The severe decrease in nitrogenous compounds
and total phosphorous in the CHU12 medium around the seventh
day was related to the fast assimilation by microalgae
in the medium. Afterwards, around the eleventh day, there
was a small increase of these nutrients, probably associated
to the small decrease of cells in the medium during the
senescent phase of the growth curve. With regard to the
different NPK combinations, nitrogenous compounds and
total phosphorous oscillated a lot during the period of
study.
In the present study different proportions
of NPK and CHU12 medium showed different growth reactions.
According to Servrin-Reyssac & Pletikosic (15), an
increase of nitrogen in the system with a consequent increase
in N/P values is favorable for the growth of chlorophycean
algae (15). This is in agreement with the findings for
A. gracilis.
The results indicate that NPK in the
20:5:20 ratio can be used directly as a good alternative
for the mass cultivation of A. gracilis, and indirectly
in the cultivation of zooplanktonic species, as it provides
good results in relation to the growth and nutritional
value of algal cells.
ACKNOWLEDGMENTS
We would like to thank FAPESP (São
Paulo State Foundation for Research Support) for the grants
given to L.C. Pelicione and A. Olivera (numbers 94/1383-8
and 96/5897-1 respectively). We would also like to thank
Silvia R.L. de Laurentiz for her help in the field and
laboratory work.
--------------------------------------------------------------------------------
RESUMO
Utilização de fertilizante
inorgânico (NPK) e do meio CHU12 no cultivo de Ankistrodesmus
gracilis em laboratório. Uma espécie de
alga clorofícea, Ankistrodesmus gracilis, foi cultivada
em dois meios de cultura, o CHU12 e em um meio alternativo
de baixo custo, o adubo químico NPK em diferentes
combinações 20:5:20; 4:14:8; 12:6:12 e 10:10:10.
Os resultados indicaram um crescimento similar nos meios
CHU12 e no NPK na proporção 20:5:20 porém,
nas outras
proporções, o crescimento
das algas foi menor. As variáveis abióticas
como alcalinidade, carbono inorgânico e nutrientes
foram similares nos tratamentos com NPK, em concentrações
menores no meio CHU12, com exceção da condutividade
e nitrato. Os resultados obtidos demonstraram que o meio
NPK na combinação 20:5:20 pode ser utilizado
diretamente no cultivo da alga clorofícea Ankistrodesmus
gracilis.
Palavras Chave: cultivo, alga, Ankistrodesmus gracilis,
NPK, CHU12, parâmetros limnológicos
--------------------------------------------------------------------------------
REFERENCES
Alfonso, E. ; Mariel, C.N. Uso de nuevos
fertilizantes para cultivar fitoplancton. Revista de Investigaciones
Marinas, VI (1):3-14, 1985.
Alfonso, E.; Martínez, L. Cultivo
de fitoplancton marino com fertilizantes inorganicos comerciales.
Revista de Inestigaciones Marinas, 13(1):81-86 , 1992.
Bosstrom, B. Factors controlling the
seasonal variations of nitrite in Lake Erken. Int. Revue.
Ges. Hydrobiol., 66:821-836 ,1981.
Botrell, H.H.; Duncan, A.; Gliwicz, Z.M.;
Grygierek, E.; Herzig, A.; Hillbricht-Ilkowska, A.; Kurasawa,
H.; Larsson, P.; Weglenska, T. A review of some problems
in zooplankton production studies. Norw. J. Zool., 24:419-456,
1976.
Carrick, H.J.; Schelske, C.L.; Aldridge,
F.J. Assessment of phytoplankton nutrient limitation in
productive waters: Application of dilution bioassays.
Can. J. Fish. Aquat. Sci., 50:2208-2221, 1993.
Chu, S.P. The influence of mineral compositon
of the medium of the growth of the planktonic algae. J.
Ecol., 30:284-325, 1942.
Golterman, H.L.; Clymo, R.S.; Ohnstad,
M.A.M. Methods for physical and chemical analysis of freshwater.
Blackwell Sci. Public. London, 1978, 213 p.
Howell, B.R. Marine fish culture in Britain
VIII: A marine rotifer, Brachionus plicatilis Müller,
and the larvae of mussel, Mitylus edulis L., as food for
larvae flat - fish. J. Cons. Int. Explor. Mer., 35:1-6,
1973.
Koroleff, F. Determination of nutrients
in: Grasshof, K. (ed.). Methods of seawater analysis.
Verlag Chimic Weinhen, New York, 1976, p. 117-181.
Mackereth, F.J.H.; Heron, J.; Talling,
J.F. Water analysis: Some revised methods for limnologists.
Freshw. Biol. Assoc., London, 1978, 119p.
Margaleff, R. Limnologia. Ediciones Omega,
Barcelona, 1983, 1010 p.
Moss, B. The influence of environmental
factors on the distribution of freshwater algae: An experimental
study. II. The role of pH and the carbon dioxide - bicarbonate
system. J. Ecol., 61(1):157-177, 1973.
Reynolds, C.S. The ecology of freshwater
phytoplankton. Cambridge University Press, London, 1984,
384 p.
Rocha, O.; Duncan, A. The relationship
between cell carbon and cell volume in freshwater algal
species used in zooplanktonic studies. J. Plankton. Res.,
7:279-294, 1985.
Servrin-Reyssac, J.; Pletikosic, M. Cyanobacteria
in fish ponds. Aquaculture, 88:1-20, 1990.
Sipaúba-Tavares, L.H.; Rocha,
O. Cultivo em larga escala de organismos planctônicos
para alimentação de larvas e alevinos de
peixes: I - Algas clorifíceas. Biotemas, 6(1):93-106,
1993.
Stein, J. Handbook of phycology methods:
Culture methods and growth measurements. Cambridge University
Press. London, 1973, 447 p.
Vollenweider, R.A. A manual on methods
for measuring primary production in aquatic environments.
Blackwell Sci. Public. London, 1974, 225 p.
Wetzel, R.G.; Likens, G.E. Limnological
analyses. Springer Verlag. New York, 1991, 391p.
Winberg, G.G. Methods for estimation
of production of aquatic animals. Academic Press. London,
1971, 175p.

