Voltar
CHEMICAL COMPOSITION OF MOUNDS OF Nasutitermes
SP. (ISOPTERA: TERMITIDAE) AND OF THE ADJACENT SOIL.
Maria Luisa T. Buschini 1, Ana Maria C. Leonardo2
1Departamento de Ecologia, Universidade Estadual Paulista,
Rio Claro - SP - Brazil
2 Departamento de Biologia, Universidade Estadual Paulista,
Rio Claro - SP - Brazil.
ABSTRACT
The influence of Nasutitermes sp. on
the dynamics of nutrients in different areas of the nest
and the surrounding soil was studied. Samples of the upper,
middle and lower area of 5 nests were collected, as well
as of the soil immediately below and beside each mound,
at a distance of 50 cm. The contents of organic carbon
and of the main mineral macro-elements, were measured
as well as the pH and the text of aluminium. A multivariate
analysis of variance (MANOVA) was carried out with these
data, and the results showed that the amounts of these
elements in the mound are larger than in the adjacent
soil, with the largest values found in its middle area.
Key Words: Isoptera, Nasutitermes, mounds, soil, mineral
concentration
--------------------------------------------------------------------------------
INTRODUCTION
The productivity of an ecosystem depends
on the amount of nutrients stored in the vegetation, in
the litter, in the soil and in animal biomass, as well
as of the transfer of nutrients among them. Nutrient cycling
in all the ecosystems is affected by a combination of
biological and physical factors. However, the relative
importance of these factors varies considerably among
the ecosystems as a result of differences in climate types,
soil, vegetation, and management practices (5).
The mineralization of organic matter
by fire, the loss of nutrients present in the soil through
the lixiviation and/or erosion and the loss due to atmospheric
factors are physical factors responsible for the nutrient
cycling (5). As biological factor stands out the microorganisms
(bacteria and fungus), which assume a predominant role
in the mineralization process.
Studies accomplished in temperate areas
demonstrated that the direct contribution of invertebrates
in the organic decomposition is small (less than 10%)
when compared with the contribution of the microbial populations
present in the soil (16, 18).
In semi-arid tropical environments, primary
decomposition is largely a function of several species
of detritivorous arthropods and, in several areas, they
have an important role in the nutrient cycling (19). As
they have true ability to modify the microenvironment
inside their nests and in their foraging galleries (9),
termites are considered some of the most efficient detritivorous
insects in dry tropical areas (5). Like ants, termites
differ from many organisms present in the soil because
they modify their microenvironment, creating, in this
way, a more favourable environment. These insects are
abundant and they play an important role in several ecosystems
(21). Through their nutritional dynamics, termites also
play an important role in the recycling of cellulose-rich
material (6).
When compared with the nests of others
insects, termite nests present the largest structural
complexity and, in most of the species, the population
is found in its interior together with the brood and the
royal pair (Desneux 1948, 1952 apud 20). For the construction
of their nests, termites use soil, faeces and saliva in
varied proportions. During this process, termites select,
transport and rearrange the particles of the soil, cementing
each other together with the organic matter. Based on
the use (or not) of these materials in the construction
of the termites nests, it was recognized different structures
of nests (7). This structural diversity results in the
transport of soil from deep and/or superficial horizons
for the local where the nests and galleries will be built,
as well as for the areas where the termites will feed.
According to Lepage (1974b) apud (21),
Macrotermes subhyalinus Rambur transports annually to
the soil surface 2000kg/ha. Thus, termites can cause a
physical disturbance in the soil profiles, a change in
its texture, as well as in the nature and distribution
of organic matter and of nutrients (20). Most of the termite
nests have more organic matter than the soil used for
their construction, because they use organic materials
(saliva or faeces) to cement their particles (2, 7).
This research had as objective the study
of the influence of Nasutitermes sp. on the dynamics of
the nutrients in different areas of the nest and in the
soil where they are distributed.
MATERIAL AND METHODS
This research was carried out in a Cerrado
area in the Municipality of Itirapina - Brazil (22°
15’ S and 47° 49’W, altitude of 765 meters).
The word Cerrado is a Portuguese term meaning "half-closed"
or "dense". In Brazilian terminology it also
describes a particular kind of vegetation similar to savannah,
although having much broader physiogonomic variation in
size and density of trees. That is, the word Cerrado would
describe a gradient of vegetation comprising "savannah
grassland", "low trees and shrub savannah"
and "savannah woodland". It is important to
point out that the similarities to savannah are only physiogonomic
and not floristic. It covers about 2 million km2, or 25%
of the whole Brazilian territory (3).
The studied area is an alluvial plain
covered by a sandy sediment, with a deep, quartzes' sand
soil type (15). Mean annual rainfall is 1425mm, with the
rain season extending from October to March, when 84%
(1199mm) of the precipitation occurs. The most rainy months
are December, January and February, with precipitation
values equal to 288, 266 and 262mm, respectively. The
driest months are July and August, with 16 and 19mm of
precipitation, respectively. The mean annual temperature
is 19.7° C, with January and February being the hottest
months with respective mean values of 22.2 and 22.3°
C. The coldest months are June and July with respective
mean temperatures of 16.4 and 16.2° C.
Due to the difficulties in determining
the Isoptera, mainly the genus Nasutitermes, the species
studied in this paper was just identified till genus even
after being enquiries to Brazilian taxonomists and abroad
Dr. Reginaldo Constantino (University of Brasilia - Brazil)
compared specimens of this work to all types of the American
Museum, and he concluded that it is very
close to Nasutitermes ephratae Holmgren.
But, according to Dr. Kumar Krishna (New York Natural
History Museum), it is very close to Nasutitermes feytaudi
Holmgren from Diamantina (MG, Brazil). In face of this,
we deposited reference specimens in the Museu de Zoologia
da USP (MZUSP 9921) at São Paulo capital city.
Since the nests of Nasutitermes sp.
have the spherical calotte shape (Figure 1), samples of
the upper, middle and lower area of 5 nests were collected.
Samples from each area corresponded to the treatments
1, 2 and 3, respectively. Samples of the soil immediately
below each mound (treatment 4) and of the adjacent soil
at a distance of 50cm, to a depth of 1 meter (treatment
5), were also collected. This protocol was necessary because
we has no knowledge of which horizon the particles used
by the termites were being removed.
The chemical analyses of mound samples
as well as of the soil helped to determine the content
of organic carbon and of the main mineral macro-elements,
being also measured the pH and the text of aluminium (12).
The analyses were made in the Laboratory of Geology of
UNESP - Rio Claro (Brazil). With these data, a multivariate
analysis of variance (MANOVA) was used, because, there
is more than one response variable (Ca, Mg, pH, P, Na,
K, Al, Co, H+) to be analysed (10).
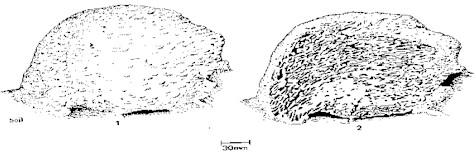
Figure 1- Nest of Nasutitermes sp. External (1) and Internal
(2) view.
RESULTS
The results of the chemical analyses
of the mounds and of the soils can be seen in Table 1.
The value of Wilkens' Lambda obtained (l = 0.001; F =
9.457), approximated by the F distribution, was highly
significant, indicating that there are differences among
the established groups. Later on, it was made the multiple
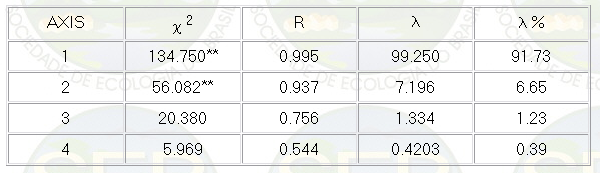
discriminant analysis or the canonical variables analysis,
through which it was observed that the factors 1 and 2
explained, respectively, 91.73% and 6.65% of the variation
among the groups (Table 2). Only these first two factors
were significant by the test of Bartlett (10).
Table 1- Mean values and standard deviations (in parentheses)
of the chemical composition of the soil and of the mound.
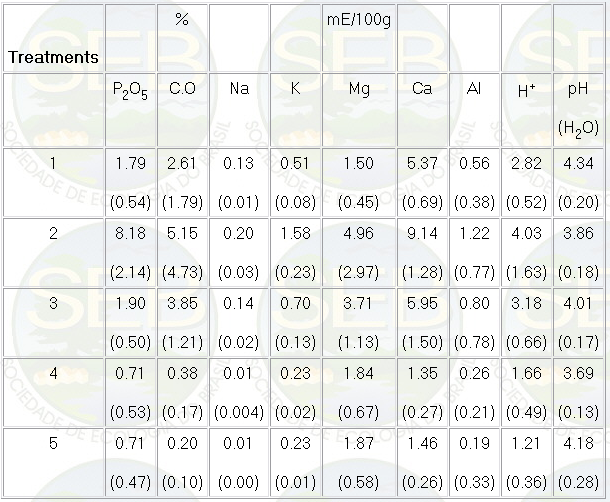
Table 2- Canonical variable analysis.
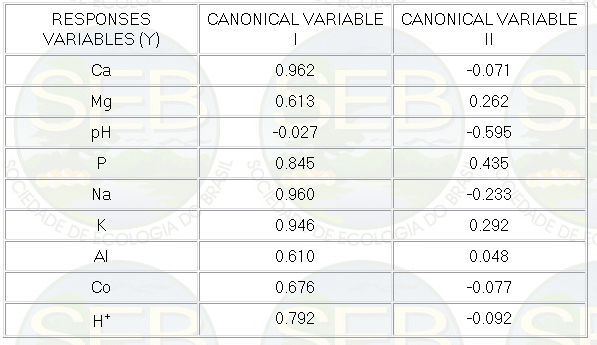
The analysed variables exercised influence on the differences
among the groups, being the variables Ca, Na, K and P
highly correlated with the canonical factor 1, and the
variable pH more correlated with canonical factor 2 (Table
3).
Table 3- Correlation among the dependent variables and
the canonical factors I and II.
In the dispersion scores of the canonical
factor 1 against the canonical factor 2 (Figure 2), it
can be seen that the treatments 1 and 3 differed from
the treatments 2, 4 and 5 with respect to both canonical
factors. These same treatments seem to present a slight
difference from each other. The treatment 2 only presented
differences in relation to the other treatments as a function
of the canonical factor 1. Faced the canonical factor
2, it only presented differences in relation to treatments
1 and 3. There was a sobreposition of the treatments 4
and 5, that is, they did not present any difference to
each other in relation to both canonical factors.
Faced with these results, it can be
seen that the middle region of the mound (treatment 2)
presented amounts of organic matter, aluminium and macro-elements
significantly larger (higher scores in factor 1) than
the other areas. In the soil samples (treatments 4 and
5), the amounts of these nutrients were significantly
smaller than in the three regions of the nests. Intermediary
amounts of these elements were found in the upper and
lower parts of the mound.
With respect to the pH, the middle region
presented a pH a little higher than that of the soil;
there was not significant difference among them. Comparing
the pH of these areas with those of upper and lower areas
of the mound, it was noticed that there was a significant
difference among them (Figure 2). The largest pH found
corresponded to the upper area of the nest (Table 1).
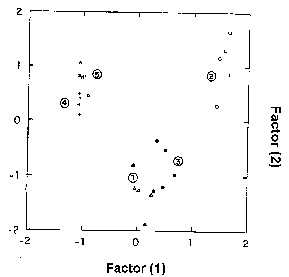
Figure 2- Canonical factors I and II for each treatment:
1, 2 and 3 correspond to the upper, middle and lower regions
of the mound, respectively. Treatments 4 and 5 correspond
to the soil immediately below the mound and the soil down
to a depth of one meter.
DISCUSSION
Like the nests of other species, the
nests of Nasutitermes sp. present concentrations of nutrients
larger than that of the soil, with the largest and smallest
concentrations occurring in the central and superior areas
of the mounds, respectively. The fact that the superior
area of the mounds present smaller amounts of nutrients
may be due to the larger rate of lixiviation suffered
by this region. The central area in termite’s nests
is constantly re-worked (7). In the nests of Nasutitermes
triodiae Froggatt, the concentrations of organic carbon
in this area, in relation to the soil from which it was
built, are larger (10%) than those of less worked areas,
as the galleries (2.7%).
Through chemical studies of macronutrientes
in nests of four species of termites, it was observed
that the nests of Nasutitermes minimus Holmgren and, especially,
of Nasutitermes surinamensis Holmgren, presented the largest
concentrations of organic matter and of inorganic nutrients
(1). According to this author, in the nests of N. surinamensis,
these results were obtained not only because this species
feeds on relatively dense wood, but also because the nests
are arboreal, impermeable to rainwater and without proliferation
of roots.
Contrary to the nests of N. surinamensis,
the nests of Nasutitermes sp. are epigeal, with reasonable
amount of roots in the inferior part of the nest. The
largest average amount of Ca found in this work was of
6.7 and 6.2 times smaller than the average amount found
immediately below in the soil and of the soil beside the
mounds, respectively. In the nests of N. surinamensis,
the average amount of Ca was 100 times superior to the
amount found in the soil of the area (1).
The concentration of nutrients in the
nests of termites results from the foraging activity of
these insects and of the use of faeces and saliva as cement
of the particles of the soil, used in the construction
of its nests (14). Gnathamitermes perplexus Banks and,
particularly, Heterotermes aureus Snyder increase the
level of organic carbon considerably in the surface of
the soil as a result of their alimentary activity, as
well as from the habit of depositing saliva and faeces
together with the particles of the soil (13). It was observed
that Nasutitermes ephratae controls not only the level
but also the distribution of P in the soils of the savannah,
being the concentration of these nutrients larger in the
nests of this species than in the adjacent soil (8).
The nests of most of termite species
generally have a pH lower than that of the soil, and similar
or slightly lower than that of the superficial soil (21).
In this experiment, although the differences among the
pH values have not been significant, it was noticed that
the pH of the central area, where there is a high concentration
of organic matter, was smaller than the pH of the superior
and inferior areas of the nest. Moreover, the pH of the
central area was also
smaller than the pH of the soil down
to a depth of one meter, but equal to that of the soil
immediately below the mound.
A logical consequence of the concentration
of nutrients in mounds, or in nests of other animals,
would be the shortage of these elements in the neighbourhood.
In this regard, a soil of low fertility becomes still
poorer after the activity of construction of the mounds,
whilst with localized points of high fertility (1). High
concentrations of nutrients in nests of several species
of termites, in Venezuela, were found (17). Those authors
concluded that these have importance for the establishment
of plants, partly due to the improvement of conditions
for seed, also favouring a larger vegetal diversity.
It was observed that nests of Armitermes
neotenicus Holmgren contain high amounts of organic matter,
coming from the faeces of this termite, with many roots
inside the nest. The roots developed better inside the
mounds because this material has larger capacity of water
retention and its disponibility of nutrients is larger
than that of the soil (11). This same author believes
that the termites, on the other hand, feed on the juice
of these roots.
In the area of the Serra of Cipó
- Minas Gerais (Brazil), where Paepalanthus bromelioides
seems to be endemic, it was found association, probably
a mutualistic one, between termites nests and the above
mentioned plant (4). According to those authors, the soils
of this area are shallow and poor in nutrients and organic
matter, suffering constant action of the fire. In this
way, P. bromelioides would supply a more amenable climate
than the adjacent environment, with the continuous renewal
of food for those insects. The mounds, on the other hand,
besides present larger concentration of nutrients, can
attenuate the degree of exhibition of P. bromelioides
to the fire.
Although the studied area suffers the
action of the fire annually and its soil is sandy, poor
in nutrients, it is believed that the mutualistic association
of this sort does not exist, because few nests of Nasutitermes
sp. present grasses in its interior. Moreover, a larger
concentration of plants was not observed in the proximity
of the mounds of this species. What was being constantly
observed is the presence of live roots in the inferior
area of the mounds, the one with the second largest concentration
of nutrients.
In high concentrations, the aluminium
can be toxical for the plants (Etherington 1975 apud 1).
Faced with this consideration, it is believed that the
non-existence of plants inside the mounds of Nasutitermes
sp. and in its proximity it is not due to this factor,
since the concentration of this element did not suffer
larger increments, when compared with the other nutrients.
ACKNOWLEDGMENTS
We would like to thank Prof. Dr. Miguel
Petrere Jr. for the suggestions and to Prof. Edson Gomes
de Oliveira for the orientation and help in the collection
of the material. Thanks are also due to Profa. Dra. Maria
M. Torres for the chemical analyses and to Prof. Dr. José
Alexandre Filizola Diniz Filho for the help in the statistical
analyses. This study was supported by grants from CNPq.
--------------------------------------------------------------------------------
RESUMO
Composição química
dos ninhos de Nasutitermes sp. (Isoptera, Termitidae)
e solo adjacente. Foi estudada a influência de Nasutitermes
sp. sobre a dinâmica dos nutrientes em diferentes
regiões do ninho, e no solo circundante. Coletou-se
amostras das regiões superior, média e inferior
de 5 ninhos e do solo imediatamente abaixo de cada cupinzeiro
e do solo a 50 cm de distância. O conteúdo
de carbono orgânico e dos principais macroelementos
minerais foi determinado, sendo medidos também
o pH e o teor de alumínio. Com estes dados, realizou-se
uma análise de variância multivariada (MANOVA)
através da qual observou-se que as quantidades
destes elementos no cupinzeiro é maior que no solo,
sendo os maiores valores obtidos em sua região
média.
Palavras-chave: Isoptera, Nasutitermes, montículo,
solo, concentração mineral.
--------------------------------------------------------------------------------
REFERENCES
Bandeira, A.G. Cupinzeiros como fonte
de nutrientes em solos pobres da Amazônia. Bol.
Mus. Pará. Emilio Goeldi, série zoológica,
2(1): 39-48, 1985.
Coventry, R.J.; Holt, J.A.; Sinclair,
D.F. Nutrient cycling by mound-building termites in low-fertility
soils of Semi-arid Tropical Australia. Aust. J. Soil Res.,
26: 375-390, 1988.
Ferri, M.G. Ecologia dos cerrados. In:
4o Simpósio sobre o cerrado, Itatiaia (SP), 1976,
p. 15-36.
Figueira, J.E.C.; Vasconcellos-Neto,
J. Paepalanthus, cupins e aranhas. Ciência Hoje,
13(75): 20-25, 1991.
Holt, J.A.; Coventry, R.J. Nutrient cycling
in Australian savannas. J. Biogeogr., 17: 427-32, 1990.
La Fage, J.P.; Nutting, W.L. Nutrient
dynamics of termites. In: Brian, M.V. (ed.). Production
ecology of ants and termites. University Press, Cambridge,
1978, p. 166-232.
Lee, K.E.; Wood, T.G. Termites and soils.
Academic Press, London, 1971, 251p.
López-Hernández, D.; Fardeau,
J.C.; Niño, M.; Nannipieri, P.; Chacon, P. Phosphorus
accumulation in savanna termite mound in Venezuela. J.
Soil Sci., 40: 635-640, 1989.
Lüscher, M. Air-conditioned termite
nests. Scientific American, 205: 138-145, 1961.
Manly, B.F.J. Multivariate statistical
methods. Chapman & Hall, New York, 1994, 215p.
Mathews, A.G.A. Studies on termites from
the Mato Grosso State, Brazil. Academia Brasileira de
Ciências, Rio de Janeiro, 1977, 267p.
Mojica, F.S.; Rodriguez, L.I.O.; Muyoz,
B.M. Métodos Analíticos Del Laboratório
de suelos. (3a edicion). Instituto Geográfico "Agustin
Codazzi", Bogota, 1973.
Nutting, W.L.; Haverty, M.I.; LaFage,
J.P. Physical and chemical alteration of soil by two subterranean
termite species in Sonora Desert grassland. J. Arid Environ.,
12: 233-239, 1987.
Oliveira-Filho, A.T. Floodplain 'murundus'
of Central Brazil: evidence for the termite-origin hypothesis.
J. Trop. Ecol., 8: 1-19, 1992.
Oliveira, J.B.; Prado, H. Levantamento
pedológico semidetalhado do Estado de São
Paulo: quadrícula de São Carlos II. Memorial
descritivo. Campinas: Instituto Agronômico, 1984.
Peterson, H.; Luxton, M. A. Comparative
analysis of soil fauna populations and their role in decomposition
processes. Oikos, 39: 287-288, 1982.
Salick, J.; Herrera, R.; Jordan, C.F.
Termitaria: nutrient patchiness in nutrient-deficient
rain forest. Biotropica, 15(1): 1-7, 1983.
Seastedt, T.R. The role of microarthropods
in decomposition and mineralization process. Annu. Rev.
Entomol., 29: 25-46, 1984.
Skujins, J. Microbial ecology of desert
soils. Adv. Microb. Ecol., 7: 49-91, 1984.
Wood, T.G. Termites and the soil environment.
Biol. Fertil Soils., 6: 228-36, . 1988.
Wood, T.G.; Sands, W.A. The role of termites
in ecosystems. In: Brian, M.V. (ed.) Production ecology
of ants and termites. University Press, Cambridge, 1978,
p. 245-292.

